Abstract
BACKGROUND. Infection by SARS-CoV2 remains a worldwide health burden. Previous studies by the ERIC (European Research Initiative on CLL) showed that patients with chronic lymphocytic leukemia (CLL) have a very-high risk of severe Coronavirus disease 19 (COVID19). In particular, age, CLL-directed treatment, and cardiac failure were identified as significant risk factors of overall survival (OS). Since the beginning of 2020 different treatment strategies have been used to fight COVID19 and different SARS-CoV2 variants alternated during the pandemic, however very few studies analyzed their impact on patients with CLL.
AIMS. The aims of this study were to investigate different manifestations, treatments and outcome of COVID19 in patients with CLL/SLL (small lymphocytic lymphoma), and MBL (monoclonal B-cell lymphocytosis).
METHODS. This is a retrospective international multicenter ERIC study from Jan 2020 till May 2022. CLL diagnosis, COVID-19 management, review of medical history, and assessment of patient status were performed by local investigators following international guidelines. Ninety institutions participated in the study. Statistical analyses were conducted using R software. During the pandemic we identified 4 phases: 1st Jan 2020 - Jun 2020, 2nd Jul 2020 - Feb 2021, 3rd Mar 2021 - Dec 2021 and 4th Jan 2022 - May 2022.
RESULTS. We gathered data from 1540 patients (93.8% CLL, 4.1% SLL and 2.1% MBL), 65% males, the median age at COVID19 was 69 years, median CIRS score was 4 (IQR 2-7), 49.6% were treated in the last 12 months before COVID19, 38.8% was on treatment at COVID19 onset (BTK inhibitor-based 51.1%, BCL2 inhibitor-based 20.7%, chemo/chemoimmunotherapy 21.5%, 6.7% others). Overall, 34.4% of patients was managed at home, 49.9% needed hospitalization and 15.7% was admitted to the intensive care unit (ICU). After a median follow-up of 2.7 months, the infection was resolved in 1124/1540 (73%) cases, while 23% died and 3.6% were still under medical observation.
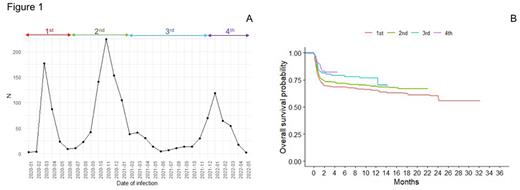
Distribution of patients among time is shown in Fig. 1A. Three hundred and four (19.8%) patients were infected during the 1st, 737 (48%) during the 2nd, 137 (8.9%) during the 3rd and 357 (23.3%) during the last 4th phase.
Comparing the features of patients infected in different phases, age at COVID19 and distribution of biological markers were similar, but more patients in the latter phases suffered from arrhythmias (8.2% vs 10.5% vs 16.1% vs 14.2%, p=0.01), chronic renal disease (3.9% vs 5.9% vs 8.9% and 11.1%, p=0.003) and other malignancies (0.3% vs 1.8% vs 2.2% vs 3.6%, p=0.04). Vaccinated patients (0% vs 0.4% vs 45.7% vs 89.1%, p<0.001) and those treated for CLL in the last 12 months (44.1% vs 44.1% vs 55.7% vs 66.1%, p<0.001) were also more common in the last phases.
COVID19 signs and symptoms differed between early and late phases, with less fever (p<0.001) and dyspnea (p=0.028) but more fatigue (p<0.001), cough (p<0.001), headache (p<0.001), anosmia/ageusia (p<0.001) and myalgia/arthralgia (p<0.001) in the later phases.
COVID19 management also changed over time, with less patients receiving a SARS-CoV2 therapy (87.6% vs 74.7% vs 78.1% vs 67.9%, p<0.001). In particular, hydroxychloroquine, azithromycin, steroids and anti-IL6/IL6R were more commonly used in the first 2 phases while antiviral and monoclonal antibodies (mAb) were mainly employed in the last 2 phases. Less patients were hospitalized (84.5% vs 67.3% vs 60.3% vs 45.8%, p<0.001), needed ICU admission (20.6% vs 17.2% vs 13.3% vs 8.1%, p<0.001) or died (29.8% vs 25.5% vs 21.8% vs 15.8%, p=0.002) through these phases. These improvements in patients' management led to an increase of OS, with 2-month OS of 70%, 74%, 81% and 83% for patients diagnosed during the 1st, 2nd,3rd and 4th phase (p=0.0015, Fig. 1B).
CONCLUSION. We analyzed one of the largest series of patients with CLL infected by SARS-CoV2 over more than 2 years of COVID19 pandemic. Patients infected during the most recent phases, though carrying a higher comorbidity burden, were less frequently hospitalized, only rarely needed ICU admission and died due to the SARS-CoV2 infection in a lower proportion compared to initial phases. The improvement in COVID-19 management with effective antiviral drugs, mAb and the high rate of vaccination together with the change in SARS-CoV2 variants over time may have played an important role in this outcome.
AV, LS, TC, AK, CD and GK equally contributed. KS and PG are both last authors.
Disclosures
Visentin:Janssen, Abbvie, CSL Behring, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Scarfò:AbbVie, AstraZeneca, Janssen, Beigene: Honoraria; BeiGene, Janssen: Other: Travel Grant; Octopharma: Speakers Bureau. Andres:Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Other: Travel Support. Cordoba:BeiGene: Consultancy; Janssen: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy; GenMab: Consultancy; Takeda: Consultancy; Kite: Consultancy, Speakers Bureau; Pfizer: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; Celgene: Honoraria; Gilead: Honoraria; Lilly: Consultancy; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Doubek:AbbVie: Honoraria; AOP Orphan: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Janssen: Honoraria. Hernandez:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; Lilly: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz: Membership on an entity's Board of Directors or advisory committees; Rovi: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Iyengar:Janssen: Speakers Bureau; AbbVie: Other: conference support; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: conference support, Speakers Bureau; Lilly: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Kite Gilead: Membership on an entity's Board of Directors or advisory committees. Itchaki:AstraZeneca: Consultancy; Janssen: Consultancy; Abbvie: Consultancy. Jaksic:Astra Zeneca: Consultancy, Speakers Bureau; Abbvie: Speakers Bureau; Johnson and Johnson: Speakers Bureau. Janssens:Genmab: Current Employment; Abbvie: Consultancy, Other: Travel Grants, Speakers Bureau; Amgen: Consultancy, Other: travel grants, Speakers Bureau; Astra-Zeneca: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau; Sanofi Genzyme: Consultancy, Speakers Bureau; Roche: Consultancy; Celgene: Other: travel grants. Kater:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Patents & Royalties: pending, Research Funding; Roche/genentech: Membership on an entity's Board of Directors or advisory committees; LAVA: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: pending; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: speakers fee, Research Funding. Levin:AbbVie, Roche and Janssen: Other: Travel expenses. Mauro:Janssen, Astra Zeneca, Beigene: Consultancy, Honoraria; Abbvie, Takeda: Honoraria, Research Funding, Speakers Bureau. Munir:Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, Abbvie, Gilead: Honoraria; Janssen, AstraZeneca, Alexion, Abbvie, Novartis, Roche: Membership on an entity's Board of Directors or advisory committees. Murru:Janssen: Research Funding; Janssen, Abbvie, Astra Zeneca: Honoraria, Other: travel. Niemann:Takeda: Consultancy; CSL Behring: Consultancy; Octapharma: Consultancy, Research Funding; Janssen: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Beigene: Consultancy; Genmab: Consultancy. Pavlovsky:AstraZeneca: Other; AbbVie: Other: Conference; AbbVie: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: Conference; Raffo: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Reda:Janssen, Abbive, Astra Zeneca, Beigene: Consultancy. Rigolin:Abbvie: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Gilead: Honoraria, Research Funding. Tadmor:Janssen: Research Funding; AbbVie, Roche, Novartis, Sanofi, Takeda, Janssen, Pfizer: Consultancy, Honoraria, Speakers Bureau. Varettoni:ABBVIE: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; ASTRAZENECA: Membership on an entity's Board of Directors or advisory committees; BEIGENE: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; JANNSEN: Membership on an entity's Board of Directors or advisory committees, Other: travel expenses. Eichhorst:Janssen, AbbVie, Lilly, AstraZeneca, BeiGene, MSD: Consultancy; Janssen, Roche, AbbVie, BeiGene, AstraZeneca, MSD: Speakers Bureau; Beigene: Other: Travel Support; Janssen, Roche, AbbVie, BeiGene, AstraZeneca: Research Funding. Rambaldi:Roche: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Kite-Gilead: Honoraria; Jazz: Honoraria; ABBVIE: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Janssen: Honoraria; Celgene-BMS: Honoraria; Omeros: Honoraria. Stamatopoulos:AbbVie: Research Funding. Ghia:BMS: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Roche: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Lilly/Loxo: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal